No injectable glutathione or skin lightening agents have been approved for use – FDA.
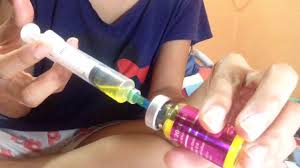
The Food and Drugs Authority (FDA) has indicated that no injectable glutathione or injectable skin lightening agents have been approved by the Authority for use in the country.
In a press statement signed by Chief Executive Officer, Delese A. A. Darko, the Authority noted that it has become aware of the promotion of such products on social media, hence the need to draw the attention of the public.
“The Food and Drugs Authority has noted with concern and cautions the public on the unsafe use of injectable glutathione which is being promoted on social media for skin lightening and bleaching, correction of uneven skin tone and reduction of blemishes.”
“So far there is no clinical evidence on the safety and effectiveness of these products and no published guidelines for correct dosing and duration of treatment,” it added.
According to the FDA, the use of injectable glutathione poses significant safety risk to its users.
Persons who use such products, the Authority said can suffer from “toxic effects on the liver, kidneys and nervous system and a serious skin reaction known as Stevens Johnson Syndrome.”
Also, users risk being infected with skin cancer.

Buttressing on why such products are not safe for public use, FDA emphasized that unapproved products which would not have been properly evaluated by the Authority may be improperly manufactured and may contain unknown harmful ingredients.
“Apart from the dangers of the product itself, administering it under unsterile conditions or by people who are not certified to administer injectable preparations is unsafe and may lead to transmission of diseases such as HIV, Hepatitis C and B.”
In view of this, the Food and Drugs Authority has advised against buying of injectable medicinal products online or from unauthorized professionals.
“The public is also urged to report the sale of glutathione injectable products or unregistered products to the FDA,” it concluded.


